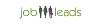Stipendio medio: euro46.952 /annuale
Più statisticheRicevi le nuove offerte di lavoro via email
- A leading clinical research organization in Milan is seeking a CRA II - III to oversee clinical trials. This position requires a university degree in life sciences, at least 2-3 years of clinical trial monitoring experience, and a strong knowledge of ICH GCP Guidelines....ConsigliatoLavoro da casaOrario flessibile
- ...innovation and excellence, and we welcome you to join us on our mission to shape the future of clinical development. As a Senior CRA / Clinical Site Manager you will be joining the world’s largest & most comprehensive clinical research organisation, powered by healthcare...ConsigliatoContratto con partita IVARemotoOrario flessibile
- A leading clinical research organization in Milan is seeking a Clinical Site Manager to oversee multiple clinical trials in Oncology. The ideal candidate will have a Bachelor's degree and over 4 years of relevant experience. Responsibilities include monitoring clinical ...Consigliato
- ...Certification in compliance with Ministerial Decree of 15 November 2011 (Official Gazette no. 11 of 14/01/2021). Experience as a CRA and/or Study Coordinator in clinical trials. Previous experience in the field oncology and onco-haematology, but not limited to this...ConsigliatoDisponibilità immediataLavoro ibridoRemoto
- ...gain the trust of new customers and secure continued growth of the company. We’re expanding our team in Italy and looking for a new CRA (CRA II - III, depending on experience) . Whether you’re building your expertise as a CRA I or ready to step into a higher CRA role,...ConsigliatoLavoro da casaOrario flessibile
- ...Qualifications: University Bachelor's Degree and Master's Degree in scientific discipline or health care In possession of CRA Certification as required by Ministerial Decree dated 15.11.2011 Experience in Pharma Industry, and/or Clinical Trials environment...ConsigliatoTempo pienoOrario flessibile
- ...and supporting research activities to achieve project objectives. ONLY CV WITH DM 15/11/11 CERTIFICATION AND PROVEN EXPERIENCE AS CRA (MINIMUM 3 VISIT IN AUTONOMY ) WILL BE TAKEN INTO CONSIDERATION. Qualifications Proficiency in Clinical Research Associates’ responsibilities...ConsigliatoTempo pienoLavoro ibrido
- A leading biomedical research company in Milano is seeking a Clinical Research Associate to manage clinical trials within a multidisciplinary team. This hybrid role combines remote work with office presence and requires at least 2 years of experience, knowledge of GCP guidelines...ConsigliatoLavoro ibridoRemoto
- ...requirement.Qualifications* University Bachelor's Degree and Master's Degree in scientific discipline or health care* In possession of CRA Certification as required by Ministerial Decree dated 15.11.2011* Experience in Pharma Industry, and/or Clinical Trials environment*...ConsigliatoLavoro da casa
- ...CRA I - ICON Biotech ICON plc is a world-leading healthcare intelligence and clinical research organization. We’re proud to foster an inclusive environment driving innovation and excellence, and we welcome you to join us on our mission to shape the future of clinical...ConsigliatoImpiego permanenteContratto con partita IVAOrario flessibile
- ...CRA II / Senior CRA ICON plc is a world-leading healthcare intelligence and clinical research organization. We’re proud to foster an inclusive environment driving innovation and excellence, and we welcome you to join us on our mission to shape the future of clinical development...ConsigliatoImpiego permanenteContratto con partita IVAOrario flessibile
- ...accuracy will improve health outcomes that people and communities depend on – now and in the future. This is a great opportunity for CRA's currently working within the life science sector for pharmaceutical companies, biotech companies or CRO's to work with a world-...ConsigliatoLavoro da remotoDisponibilità immediataOrario flessibile
- ...In-House CRA ICON plc is a world-leading healthcare intelligence and clinical research organization. We’re proud to foster an inclusive environment driving innovation and excellence, and we welcome you to join us on our mission to shape the future of clinical development...ConsigliatoImpiego permanenteContratto con partita IVALavoro ibridoOrario flessibile
- ...in scientific discipline or health care • Requires at least 3 years of year of on-site monitoring experience • In possession of CRA Certification according to Ministerial Decree dated 15.11.2011 • Good knowledge of, and skill in applying, applicable clinical...ConsigliatoTempo pieno
- ...richieste diverse soft skill ed esperienze. La preghiamo di consultare attentamente la panoramica riportata di seguito. Sponsor Dedicated CRA II - Home Based ITA Syneos Health® is a leading fully integrated biopharmaceutical solutions organization built to accelerate...ConsigliatoLavoro da casaRemoto
- Sanipiù Pro-Divisione Specialistica Sanità del Gruppo Lavoropiù-ricerca: Biologo/a PMA– Embriologo/a PMA per una prestigiosa casa di cura privata a Milano, in una zona facilmente raggiungibile dai mezzi. Requisit i richiesti: Laurea in Biologia o Biotecnologie...24 h/sett.Part-time
- ?? Manpower cerca per importante multinazionale farmaceutica una figura da inserire come: ADDETTO/A STRUMENTAZIONE DI LABORATORIO FARMACEUTICO La risorsa sarà inserita all'interno del laboratorio chimico e si occuperà delle seguenti attività: ?? Responsabilità principali...Turni
- Sei un perito chimico o un operaio con esperienza in aziende alimentari, chimiche, farmaceutiche e ti piacerebbe lavorare come addetto/a laboratorio analisi in produzione? Allora candidati subito! Stiamo cercando, per azienda specializzata nel settore chimico di Corsico...Tempo pienoTurniDal lunedì al venerdìTurno di notte
- Senior Medical Director, Italy page is loaded## Senior Medical Director, Italylocations: Milantime type: Full timeposted on: Posted Yesterdayjob requisition id: R32385BeOne continues to grow at a rapid pace with challenging and exciting opportunities for experienced professionals...Stage/Tirocinio
- A leading pharmaceutical company is seeking a Senior Medical Director in Milan. This role involves overseeing medical affairs, leading the local team, and developing strategies for oncology products. Candidates must have an M.D. degree and over 12 years of experience in...
- Orienta Spa, Agenzia per il lavoro filiale di Milano Giotto, ricerca per importante azienda nel settore chimico / farmaceutico con sede a MILANO SUD: un/a ANALISTA LABORATORIO CONTROLLO QUALITA' PRODOTTI FINITI Il candidato deve occuparsi di Analisi di...Tempo pienoTurniDal lunedì al venerdì
- Sanipiù Pro- Divisione Specialistica Sanità del Gruppo Lavoropiù- ricerca: Tecnico/a di laboratorio biomedico per una rinomata azienda ospedaliera presso un loro laboratorio di analisi cliniche nella zona di Paderno Dugnano (MI). Requisiti richiesti: Laurea ...Tempo pienoTurni
- Un'agenzia di PR internazionale sta cercando un Client Director a Milano. Il candidato ideale avrà un'esperienza di 8-10 anni nel settore PR, con focus in healthcare e life sciences, e sarà responsabile della definizione di strategie integrate di comunicazione e gestione...Smart working
- La risorsa farà parte del Laboratorio di Controllo Qualità chimico e si occuperà dell'esecuzione delle determinazioni analitiche chimico fisiche e quantitative sulle materie prime in ingresso. Utilizzerà strumentazione analitica di vario livello di complessità (HPLC, ...
- Job Summary Medpace is currently seeking candidates with PhDs and/or Post-Doctoral Research experience for a full-time, office-based Associate Clinical Trial Manager (aCTM) to join our Clinical Trial Management team. The aCTM will be a part of the Clinical Trial...Tempo pieno
- A leading biopharmaceutical company is seeking a Sponsor Dedicated Clinical Trial Manager based in Milano. This role requires fluency in Hebrew and previous experience with Israeli clinical processes. The ideal candidate will oversee project deliverables and manage vendor...Lavoro da casa
- sede: Milano centro Profilo ideale Laurea in campo scientifico (Medicina, Biologia, Farmacia, Biotecnologie o discipline affini) con ottimo curriculum studiorum; Esperienza nel ruolo di almeno 10 anni nel ruolo con provenienza dal settore medico/studio dentistico...Impiego permanentePart-timeTempo pienoDisponibilità immediata
- Etjca Group, filiale di Milano, cerca per importante azienda operante nel settore di oli e lubrificanti industriali, un/una: TECNICO/A DI LABORATORIO La risorsa sarà inserita nel laboratorio chimico con lo scopo di fornire supporto operativo e organizzativo, riporterà...Tempo pienoDal lunedì al venerdì
- ...processes. At least 3 years of previous experience as Clinical Trial Manager. Background & Expertise Preferably with historical CRA or strong site-level experience. Solid understanding of site operations and dynamics. Start-Up Phase Experience Involvement...Lavoro da casa
- Select how often (in days) to receive an alert: Molecular Laboratory Technician Published on: Feb 21, 2026 Location: Bresso Job Category: Research & Development Employment type: Temporary Full Time Why Join Diasorin? Impactful Work: When you join Diasorin...TemporaneoTempo pieno