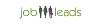Stipendio medio: euro19.826 /annuale
Più statisticheRicevere le nuove offerte di lavoro via email
- ...New product creation ~5+ Years Marketing experience in the Pharmaceutical industry ~ Scientific background an advantage ~ Medical Devices/OTC/Pharmaceutical experience ~ Dermatology and/or wound care experience advantageous ~ Fluent English This is a...Dispositivi medicale
- ...disruptions. Qualifications ~2-3 years of experience as a Medical Field Service Engineer ~ Min 2 years of experience in calibration, repair, validation, qualification for equipment (Medical device / Pharma) ~ Excellent troubleshooting and problem-solving skills...Dispositivi medicaleLavoro remoto
- ...consent decree experience, deep 21CFR Part 11 experience is highly desired Experience in biotech and pharma is preferred over medical device Experience with MES, Delta V, PI is a plus. We use all major systems as we serve a multitude of clients. Rockwell, GE,...Dispositivi medicale
- ...website. Who we are: OPIS is an International CRO with 25 years of experience in conducting Phase I-IV, non-interventional and medical devices studies on an international level. We always offer state-of-the-art information technology solutions and innovative approaches...Dispositivi medicale
- ...Job Summary We are seeking an Allergist - Medical Director to join our dynamic team in Milan. The Medical Director will have overall... ...services to the biotechnology, pharmaceutical and medical device industries. Our mission is to accelerate the global development...Dispositivi medicaleContrattoLavoro ibridoOrari flessibili
- ...Bachelor's degree in engineering, life sciences, or related field. Minimum 8 years of experience in quality management within the medical device industry. Proven experience in managing cross-functional teams in a regulated manufacturing environment. Experience with...Dispositivi medicaleContratto
- ...Product Specialist of Cosmo Intelligent Medical Devices. Work Location: Rome – Italy – Viale Ostiense 131/L Key Responsibilities:... ...experience as a Product Specialist or similar role in the medical device industry. Strong knowledge of the medical device sector,...Dispositivi medicale
- ...Requirements: Relevant work/study experience abroad Relevant work experience with an ISO certification body Experience in medical device companies is preferred Experience in clinical evaluation of a medical device is preferred Knowledge of and experience in...Dispositivi medicale
- ...effectiveness. Conduct bioburden and sterility testing on sterile devices, assessing compliance with requirements and managing any... ..., CTF). Minimum experience in diagnostic systems and in medical device companies (nice to have). Good knowledge of English (B2/C1...Dispositivi medicaleContratto a termine fisso
- ...azienda farmaceutica produttrice di soluzioni infusionali sterili, medical device e cosmetici, ha sede a Trieste ed opera in un contesto... ...private # instaurare e mantenere solide relazioni con i clienti medici / specialisti # Eventuale ulteriore esperienza di vendita in...Dispositivi medicale
- ...in some of the most challenging cancers. We are seeking a Medical Director to lead medical strategy and execution in Italy. This... ...~3 years’ experience in oncology (clinical, pharma, or medical devices) ~ Strong knowledge of Italian oncology networks and dynamics...Dispositivi medicale
- ...3 years of experience in Field Service (medical field experience preferred). Willingness... ...Service Engineer, preferably in medical devices companies Strong customer orientation... ...Electrical Engineering, Maintenance, Medical Device, Field Service, Engineering,...Dispositivi medicale
- ...leading biomedical company. WHAT YOU'LL BE DOING: Learn the medical devices’ functionality for testing the connectivity software products... ...computers and networks used as test environments for medical device connectivity software applications. Under the periodic...Dispositivi medicale
- ...Experience in a fast-paced manufacturing environment; experience in a medical environment is a plus. ~ Excellent communication, negotiation... ...Familiarity with automotive, electronics, and medical device industry standards What’s in it for you? Work for a global...Dispositivi medicaleInizio immediato
- ...and interpret results for studies in Phases I-IV and across studies for submission in various therapeutic areas, including both medical device and drug settings. KEY RESPONSIBILITIES Carry out tasks within the specified timelines and quality standards....Dispositivi medicaleOrari flessibili
- ...Procedures. Perform statistical analysis of data. Engage in continuous improvement of all Quality Management Systems related to Medical Devices. Requirements Bachelor's or Master's Degree in Engineering: Electronic, Electrical, or Biomedical. Knowledge of QMS...Dispositivi medicale
- ...and technologies to the world -- in nutrition, diagnostics, medical devices and branded generic pharmaceuticals -- that create more possibilities... ...about the structural heart therapy and MitraClip medical device for the organization. MAJOR RESPONSIBILITIES Provides...Dispositivi medicaleStage
- ...MMS is an innovative, data-focused CRO that supports the pharmaceutical, biotech, and medical device industries with a proven, scientific approach to complex trial data and regulatory submission challenges. Strong industry experience, technology-enabled services, and...Dispositivi medicaleTempo pieno
- ...repair the mitral valve Kalios is the first mitral annuloplasty device that can be percutaneously adjusted to optimize valve repair at... ...can be repeated multiple times post-operatively. Affluent Medical is currently developing investigational medical devices not yet...Dispositivi medicale
- ...Procedures. Perform statistical analysis of data. Contribute to continuous improvement of Quality Management Systems related to Medical Devices. Requirements Bachelor's or Master's Degree in Engineering (Electronics, Electrical, Biomedical) or a technical...Dispositivi medicaleContrattoTemporaneoLavoro su turni
- ...Manager in Biomedical Sector Company Context A company focused on engineering new products and lines of business related to medical device manufacturing within a multinational group operating in the biomedical field. Position Offer The selected candidate will...Dispositivi medicale
- ...Mechanical Engineer of Cosmo Intelligent Medical Devices. Work Location: Rome – Italy – Viale Ostiense 131/L Key Responsibilities:... ...in written and spoken English. Experience in the medical device industry. Knowledge of IEC 60601-1 and IEC 60601-1-2 standards...Dispositivi medicaleStage
- ...and be a driving force behind our mission to revolutionize the medical industry! Key responsibilities Your main responsibilities... ...related field Experience as a Product Manager in the medical device industry, ideally with exposure to cardiology, neurology, or anesthesiology...Dispositivi medicaleTurno di pomeridiano
- ...engineering, nursing, life sciences, or a related field. At least 1 year of experience in clinical support, healthcare sales, or medical devices—ideally in endovascular or cardiology procedures. Solid understanding of anatomy, physiology, and clinical workflows;...Dispositivi medicale
- ...contract research organization (CRO). We provide Phase I-IV clinical development services to the biotechnology, pharmaceutical and medical device industries. Our mission is to accelerate the global development of safe and effective medical therapeutics through its...Dispositivi medicaleContrattoLavoro remotoOrari flessibili
- ...Azienda in grande crescita Azienda Il cliente è una PMI italiana attiva nella produzione e commercializzazione di dispositivi medici estetici (filler, skinbooster, cosmetici funzionali), con sede in Italia centrale e certificazioni CE, ISO 13485, e una rete...Dispositivi medicale
- ...compilazione e trasmissione della documentazione obbligatoria (es. Medical Device Incident Report – MIR); Pianificazione e partecipazione a... ...di prodotto e di sistema nel settore dei dispositivi medici in accordo alle normative ISO 13485 e MDR Conoscenza di mercati...Dispositivi medicaleContrattoTempo pienoDal lunedì al venerdì
- ...Manage the post market surveillance activities to ensure that medical devices continue tobe safe and well-performing and to ensure... ...diagnostics / medical devices At least five years of Medical Device / -IVD or chemical-pharmaceutical industry experience in similar...Dispositivi medicaleTempo pieno
- ...clients and families on available services and care plan benefits, ensuring clarity and understanding. Approve the supply of medical devices and aids, coordinating with suppliers to meet client needs. Oversee recruitment, orientation, and retention of care teams,...Dispositivi medicaleLavorare da casa
- ...Qualifications & Skills Degree in Law or equivalent, Minimum 8 years of experience in a similar role (experience in the medical device sector is a plus), Solid knowledge of tender regulations and e-procurement platforms (e.g., Sintel, MEPA), Excellent organizational...Dispositivi medicaleContrattoLavoro ibridoLavorare da casaLavoro remoto